In a year-long clinical trial, 5% of patches fully detached.
Check Twirla daily and after water exposure to make sure the edges haven’t lifted.
Meet Twirla®
Meet Twirla®

Birth control designed to fit your life
Weekly. Self-administered. Just 3 patches a month.
Twirla is a hormonal birth control patch for women with a body mass index (BMI) less than 30 kg/m2 who can become pregnant. Twirla is less effective in women with a BMI of 25 kg/m2 or more to less than 30 kg/m2. Do not use if your BMI is 30 kg/m2 or more.
The hormone combination in Twirla has a long history of use in birth control pills.
Same-Time-Every-Day Pill or Weekly Patch
Which birth control method fits your life?
You know the daily pill routine: stop and take it every day, ideally at the same time each day for at least 21 days each month.
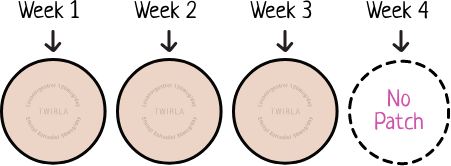
With Twirla, you change your patch just once a week for 3 weeks of your cycle. During the fourth week of your cycle, you wear no patch and should expect your period.
The most common side effects reported by women using Twirla in a clinical study were skin reactions at the patch site, nausea, headache, menstrual cramps, and weight gain. These are not all the possible side effects of Twirla.
Call your healthcare provider for medical advice about side effects.
Exclusive PATCH TECHNOLOGY
Purposefully designed—and proven—to stay in place.
Only Twirla uses Skinfusion® patch technology for consistent drug delivery and to help maintain adhesion. Twirla is made with a soft, flexible fabric designed to contour with a woman’s body.
*In the trial, subjects reported better adhesion on the abdomen than on the upper torso or buttock. Five percent of patches fully detached. Full detachments occurred more frequently for patches exposed to water.


To prevent pregnancy, Twirla needs to remain adhered to the skin for 7 days. Check it daily and after any water exposure to ensure the edges haven’t lifted. Avoid prolonged water exposure (swimming or contact with water often or for long periods of time—30 minutes or more) or the use of makeup, creams, lotions, oils, powders, or any other products on the skin area where you put or plan to put the patch.
Is Twirla right for you?
Making a decision about which birth control is right for you starts with a conversation.
Twirla may not be appropriate for everyone. To find out if Twirla is right for you, you’ll need to talk to your doctor.
